原料药
加巴喷丁 (US FDA, CEP, GMP, TGA, EU GMP)
结构式: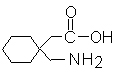 CAS NO: 60142-96-3 外观:白色或类白色晶体粉末; 含量:≥99.0%. |
Gabapentin, received Canada DMF registration number: 2006-067 in 2006
Gabapentin, received DMF registration number: 20826 of FDA in 2007
Gabapentin, receivedreceived GMP certificate No. ZK0638 in May.19,2009
Our product, Gabapentin, received TGA GMP certificate No. MI-2008-CE-00293-3 in August.14,2009
Our product, Gabapentin, received Gabapentin Korea FDA GMP Certification: 20100085654 in April.5,2011.
Our product, Gabapentin, received Gabapentin CEP certificate ,the certificate NO.:R0-CEP 2011-258-Rev 00 in August.9,2012
我公司产品加巴喷丁于2012年8月14日成功得到加巴喷丁印度注册证书,证书号:BD-1080。
我公司产品加巴喷丁于2012年10月10日成功得到加巴喷丁欧盟GMP证书,证书号:VTV/101012/3GMP-CHI 。